- Courses
- GS Full Course 1 Year
- GS Full Course 2 Year
- GS Full Course 3 Year
- GS Full Course Till Selection
- Online Program
- GS Recorded Course
- NCERT (Recorded 500+ Hours)
- Polity Recorded Course
- Geography Recorded Course
- Economy Recorded Course
- AMAC Recorded Course
- Modern India, Post Independence & World History
- Environment Recoded Course
- Governance Recoded Course
- Science & Tech. Recoded Course
- International Relations and Internal Security Recorded Course
- Disaster Management Module Course
- Ethics Recoded Course
- Essay Recoded Course
- Current Affairs Recoded Course
- CSAT
- 5 LAYERED ARJUNA Mentorship
- Public Administration Optional
- ABOUT US
- OUR TOPPERS
- TEST SERIES
- FREE STUDY MATERIAL
- VIDEOS
- CONTACT US
INDIA TAKES FIRST STEP TO REMOVE ANIMALS FROM DRUG-TESTING PROCESS
INDIA TAKES FIRST STEP TO REMOVE ANIMALS FROM DRUG-TESTING PROCESS
10-08-2023
Latest Context
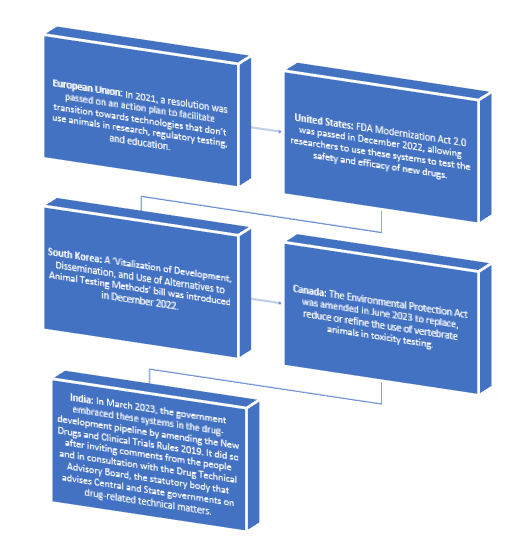
The Government approved an amendment to the New Drugs and Clinical Trial Rules (2023) to end the use of animals in research, particularly for drug testing.
Facts about Drug-Testing Process
- Every drug on the market undergoes a protracted series of testing to see if it can effectively cure the condition for which it was developed and whether it has any negative side effects.
- Testing the candidate molecule in at least two animal species—a rodent (mouse or rat) and a non-rodent, such as canines and primates—has traditionally been the initial stage in this approach.
- The amendment permits scientists to evaluate the efficacy and safety of new drugs using non-animal and human-relevant techniques such as 3D organoids, organs-on-chip, and sophisticated computational tools.
Why did the government stop using animals for drug testing?
- A lab-bred animal species raised in controlled conditions may not accurately represent the human response to a drug because humans are more complex beings whose biological processes and their responses frequently vary from person to person based on factors like age, sex, pre-existing diseases, genetics, diet, etc.
- The high failure rate of the medication development process is a reflection of this mismatch between the two species. Despite increased investment in the pharmaceutical industry, the majority of medications that successfully passed the animal testing stage fall short in the pipeline's last step of human clinical trials.
- Researchers are concentrating more on systems that are better at capturing the nuances of human biology and anticipating human reactions as a result of the constraints of the traditional testing method, which start with animals.
What are the alternative testing modes?
- Several technologies have been created over the past few decades using human cells or stem cells, including "organoids" or "mini-organs," which are millimetre-sized, three-dimensional cellular constructs that imitate certain biological organs.
- The 'organ-on-a-chip', which is a popular technology, consists of AA-battery-sized chips packed with human cells and linked to microchannels to replicate blood flow in the body. These devices record many elements of human physiology, including internal bodily impulses and interactions between tissues.
- For more than 20 years, researchers have also employed additive manufacturing methods.
- To "print" biological tissues utilising human cells and fluids as "bio-ink," researchers created the inkjet bioprinter and 3D bioprinter in 2003 by altering conventional inkjet printers.
- These systems have the potential to transform medication development and design. Considering that they can be created using patient-specific cells and used to customise drug testing.
Challenges
- Developing an organ-on-a-chip system: It typically requires multidisciplinary knowledge and expertise in materials science to find the right material to ensure that the chip does not interfere with biological processes, fluid dynamics to simulate blood flow inside the microchannels, electronics to integrate biosensors that can measure pH, oxygen, etc., engineering to design the chip, and pharmacology and toxicology to interpret the action of the drug.
- Resources for development and research: The majority of the chemicals, equipment, and materials used in cell culture are now imported from the United States, Europe, and Japan. To build an end-to-end ecosystem in India, there is a great need and potential in many different fields linked to cell culture, material science, and electronics.
- Variability in the data due to variations in procedures and competence from lab to lab. One lab would build a system using solely liver cells, while another might include immune cells in its liver-on-a-chip to investigate the immune system and liver. It implies that there cannot be a "standard" or "universal" liver-on-a-chip for the investigation of all liver disorders.
Way Forward
- On the subject of deploying new human-based technologies, multi-stakeholder groups with representatives from business, academia, government, and regulatory authorities are necessary.
- Technology developers from academia and business have suggested setting up a "Centre for Excellence" in India to bring together experts from many fields to produce preclinical human models to facilitate this communication between diverse fields of study.
- Given recent advancements in cell-based and gene-editing-based medicines, the standards on animal testing need to be reevaluated and amended.
- It's a multidisciplinary enterprise that requires specialised training and resource development, both of which the nation now lacks.