- Courses
- GS Full Course 1 Year
- GS Full Course 2 Year
- GS Full Course 3 Year
- GS Full Course Till Selection
- Answer Alpha: Mains 2025 Mentorship
- MEP (Mains Enrichment Programme) Data, Facts
- Essay Target – 150+ Marks
- Online Program
- GS Recorded Course
- Polity
- Geography
- Economy
- Ancient, Medieval and Art & Culture AMAC
- Modern India, Post Independence & World History
- Environment
- Governance
- Science & Technology
- International Relations and Internal Security
- Disaster Management
- Ethics
- NCERT Current Affairs
- Indian Society and Social Issue
- NCERT- Science and Technology
- NCERT - Geography
- NCERT - Ancient History
- NCERT- World History
- NCERT Modern History
- CSAT
- 5 LAYERED ARJUNA Mentorship
- Public Administration Optional
- ABOUT US
- OUR TOPPERS
- TEST SERIES
- FREE STUDY MATERIAL
- VIDEOS
- CONTACT US
Thallium Poisoning
Thallium Poisoning
30-10-2023
Why in News?
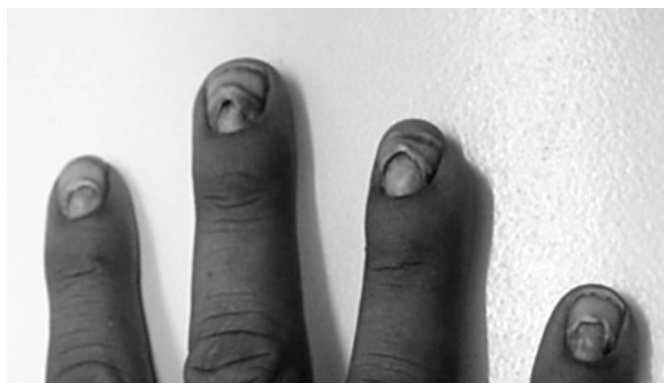
Recently, Multiple family members in Mahagaon village, Maharashtra, were exposed to thallium poisoning, a chemical that operates silently and evades detection.
Key Facts about Thallium
-
About:
- Sir William Crookes discovered Thallium (Tl), a chemical element with an atomic number of 81, in 1861.
- Thallium, a tasteless and odorless poison, has been utilized by murderers as a difficult-to-detect poison.
-
Appearance:
- A soft, silvery-white metal that tarnishes easily.
- It is a soft, heavy, inelastic metal.
-
Sources:
- Thallium, a by-product of copper, zinc, and lead refining, is found in pyrites, a type of ores used to produce sulfuric acid.
- The substance is present in trace amounts within the Earth's crust.
-
Uses:
- Thallium's use is restricted due to its toxic nature.
- Thallium sulfate, a rodent killer, is now banned for household use in many developed nations.
- The technology is utilized in the electronics industry specifically for photoelectric cells.
- Thallium oxide is utilized in the production of high-refraction glass and low-melting glass.
- This material is utilized in the production of low temperature thermometers and imitation jewels.
-
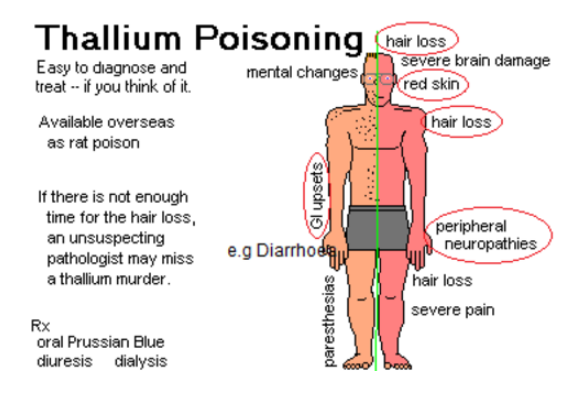
Health Hazards:
- Thallium can cause neurological damage, including headaches, weakness, and irritability, and repeated exposures can lead to tremors, hallucinations, coma, and even death.
-
Antidote:
- Prussian blue is utilized in non-radioactive thallium poisoning.