- Courses
- GS Full Course 1 Year
- GS Full Course 2 Year
- GS Full Course 3 Year
- GS Full Course Till Selection
- Answer Alpha: Mains 2025 Mentorship
- MEP (Mains Enrichment Programme) Data, Facts
- Essay Target – 150+ Marks
- Online Program
- GS Recorded Course
- Polity
- Geography
- Economy
- Ancient, Medieval and Art & Culture AMAC
- Modern India, Post Independence & World History
- Environment
- Governance
- Science & Technology
- International Relations and Internal Security
- Disaster Management
- Ethics
- NCERT Current Affairs
- Indian Society and Social Issue
- NCERT- Science and Technology
- NCERT - Geography
- NCERT - Ancient History
- NCERT- World History
- NCERT Modern History
- CSAT
- 5 LAYERED ARJUNA Mentorship
- Public Administration Optional
- ABOUT US
- OUR TOPPERS
- TEST SERIES
- FREE STUDY MATERIAL
- VIDEOS
- CONTACT US
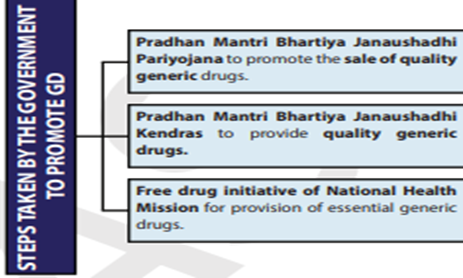
DOCTORS TO PRESCRIBE ONLY GENERIC DRUGS
DOCTORS TO PRESCRIBE ONLY GENERIC DRUGS
25-08-2023
Key Points
- The NMC has suspended a regulation from the Registered Medical Practitioner (Professional Conduct) Regulations, 2023 that would have fined doctors for not prescribing generic medications.
- A generic drug (GD) is a medicine developed to be identical to a brand-name drug that has previously been commercialized in terms of dose form, potency, mode of administration, quality, performance traits, and intended use.
- The Drugs and Cosmetics Act of 1940 and the Drugs & Cosmetics Rules of 1945 do not define "generic drugs."
- Although compulsory licensing under the Indian Patent Act permits it without consent during any urgency, it is still possible to commercialize it after the branded drug's patent expires.
-
Significance of GDs for India:
- increases the availability and accessibility of essential medications.
- Due to its generally lower pricing, it could lower healthcare costs.
- For the same product, many generic medications are frequently approved, resulting in competition.
- 20–22% of generic medicine exports worldwide come from India.
- Challenges: Lack of facilities for high-quality testing, perpetual renewal of patents, heavy reliance on imports for key raw materials, dealers in fake medications, etc.